UTI/Urosepsis

RECCE® 327 I.V.

Overview
UTI Cases and Fatalities by Geographical Area
Worldwide
Cases
400 million
200,000
deaths in 2019
USA
40%
of the women in the USA will develop a UTI at some point in their lifetime
Europe
Over
4 million
patients acquired healthcare- associated UTIs annually
Australia
Cases
250,000
annually
UTI Patient Journey
- Urinary Tract Infection (UTI) is one of the most common infectious diseases
- The most common pathogen using UTIs is Escherichia coli (E. coli) with 62%
- One in three uncomplicated UTIs in young healthy women are Bactrim-resistant
- One in five are resistant to five other common antibiotics.
- Previous years have demonstrated the likelihood of antibiotics killing most UTIs is rapidly dropping


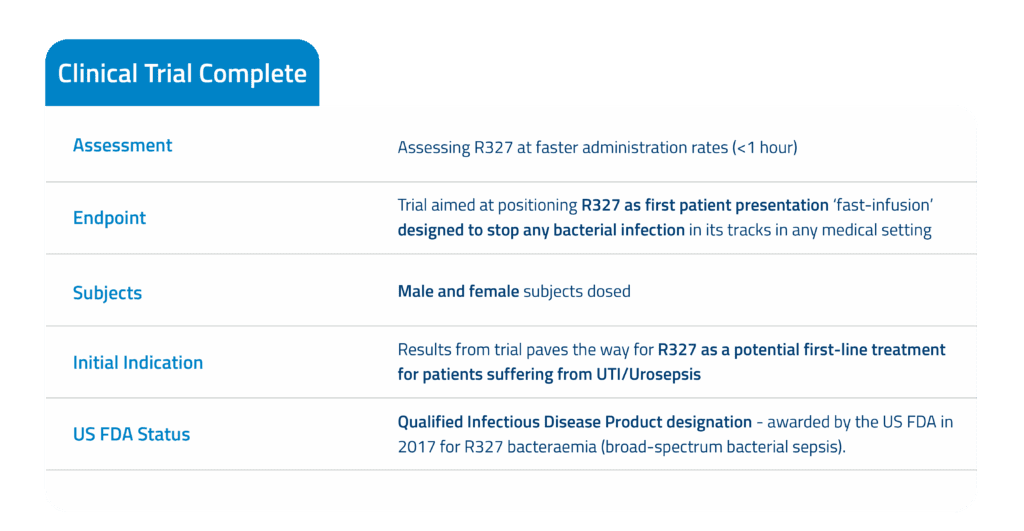
Clinical Trial Complete
Following the successful completion of its Phase I and Phase I/II clinical trials, Recce Pharmaceuticals continues to advance the development of RECCE® 327 (R327) in intravenous (I.V.) formulation for the treatment of urinary tract infections (UTIs) and urosepsis, a serious and often life-threatening complication.
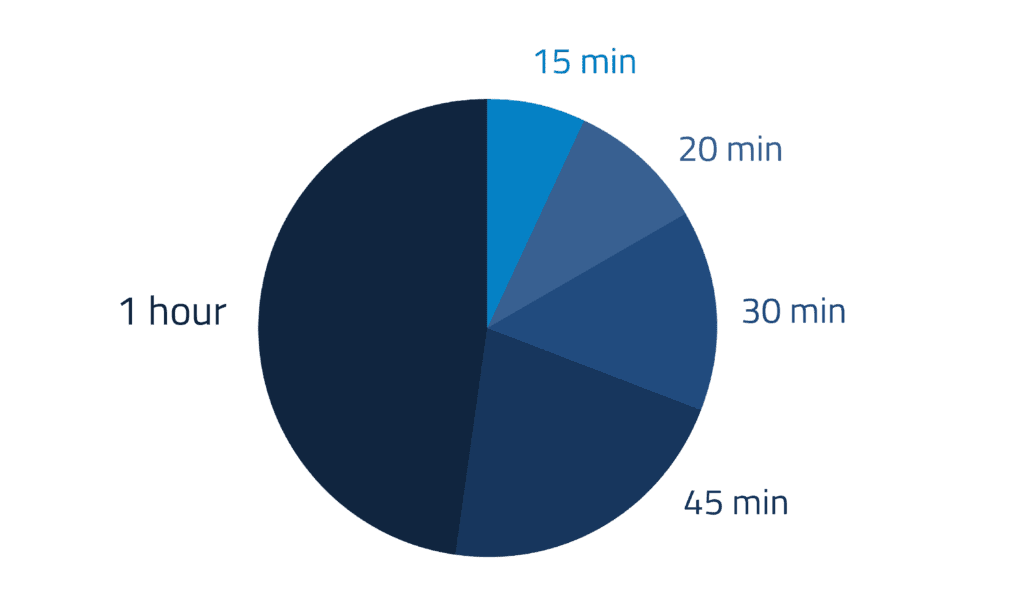
The Phase I trial demonstrated that R327 was safe and well tolerated at escalating doses up to 6,000 mg delivered via 1-hour infusion, with no serious adverse events reported. Pharmacokinetic analyses confirmed that R327 concentrates in the urinary tract, with urine levels up to 20 times higher than plasma, supporting its application as a targeted therapy for urinary pathogens.
The subsequent Phase I/II trial further validated the compound’s safety and rapid bactericidal activity in ex vivo studies. In these tests, R327-treated urine from trial participants was shown to effectively and irreversibly eliminate E. coli, a common cause of UTIs and sepsis.

About RECCE® 327
- A reduction in protein synthesis
- Depolarization of cellular membranes

