Burn Wound
Infections

- Home
- Product Candidates
- Burn Wound Infections
RECCE® 327 Topical

Overview
Burn Wound Infection Cases and Fatalities by Geographical Area
Worldwide
13 million
cases requiring medical intervention annually
180,000
deaths annually
USA
500,000
burn wound injuries requiring medical intervention annually
Europe
970 million
The burn care market in Europe is expected to reach a projected revenue of US$ 970.2 million by 2030
Australia
5,000
Approximately 5,000 burn-related hospital admissions annually
Advancing the Treatment of Burn Wound Infections
Recce Pharmaceuticals is pioneering the development of RECCE® 327 Gel (R327G), a novel synthetic anti-infective designed to revolutionise the treatment of burn wound infections.
Burn injuries are highly susceptible to infection, with rapid bacterial colonisation increasing the risk of delayed healing and progression to systemic infection and sepsis. R327G is being developed to support infection management in the acute phase of burn injury, where early intervention is critical in both military and civilian settings.
US Department of Defense Grant: Accelerating Breakthroughs
Recce has been awarded US$2 million (~ A$3 million) in funding from the US Department of Defense’s Congressionally Directed Medical Research Programs (CDMRP) under the Military Burn Research Program (MBRP). This prestigious grant highlights the recognition of R327G’s potential in the management of burn wound infections, particularly in military environments where rapid, effective treatment is vital.
Cooperative Research with the U.S. Army Institute of Surgical Research
Building on this funding, Recce has entered into a Cooperative Research and Development Agreement (CRADA) with the U.S. Army Institute of Surgical Research (USAISR), the U.S. Army’s centre of excellence for combat casualty research and burn care.
Under this agreement, R327G will be evaluated in the Walker-Mason rat model of burn wound infection, a validated preclinical model designed to replicate battlefield burn injuries and subsequent infection. The study will assess R327G’s ability to reduce bacterial burden, including against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa, two clinically significant pathogens commonly associated with burn wound infections.
Key Highlights:
- US$2 Million Funding: Supporting the development and evaluation of R327G for acute burn wound infections.
- Military-Grade Application: Designed for use in far-forward military settings, enabling rapid treatment at the point of injury.
- Potential to Replace Traditional Antimicrobials: Aiming to outperform current antimicrobial dressings and topical agents used in military healthcare.
Supporting Clinical and Preclinical Evidence
R327G has been evaluated in an exploratory Phase I/II clinical study, where patients with burn wounds were treated for up to two weeks. The study demonstrated that R327G was safe and well tolerated.
- Rat Thermal Wound Infection Model: Demonstrated significant antibacterial activity against MRSA, reduced bacterial load and higher percentage of wound closure with increasing doses of R327 compared to Soframycin.
Active Research Partnership with the U.S. Army Medical Research
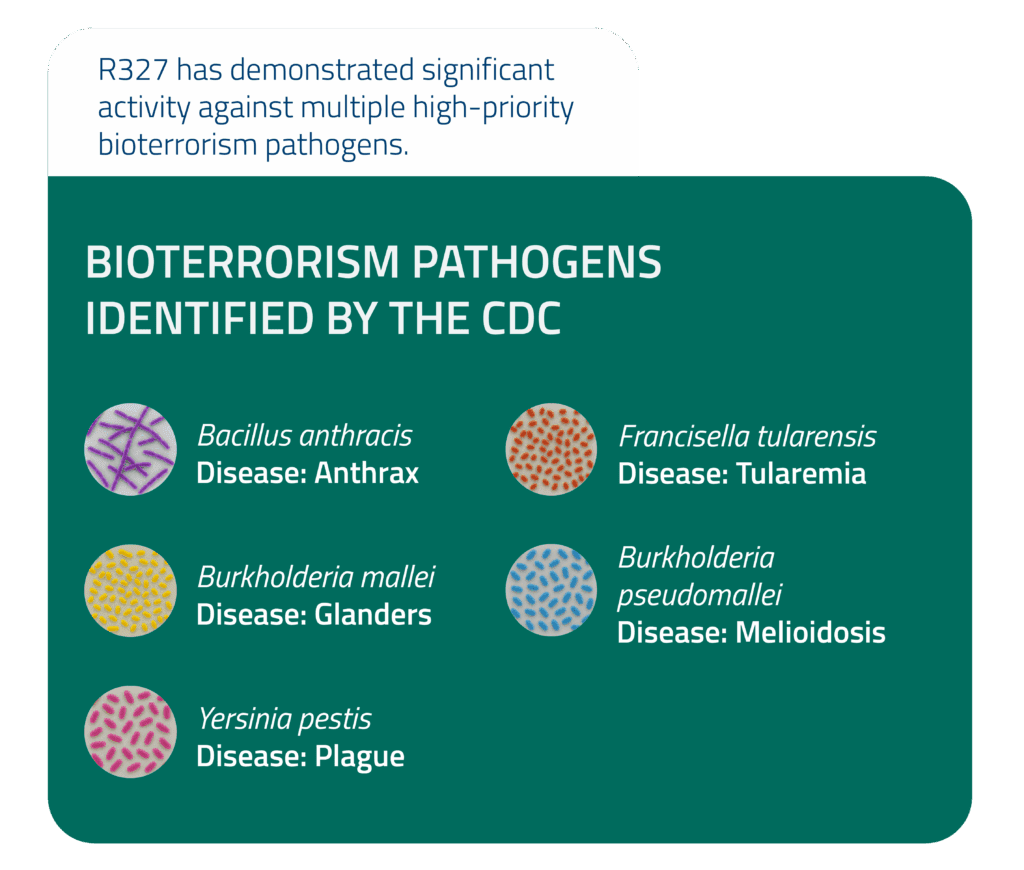
In parallel, Recce has entered into an active research partnership with the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) to test R327 against high-priority biothreat pathogens, reinforcing the platform’s dual-use potential in both combat and biodefense contexts.
Highlighting the growing recognition of its innovation, Recce was also accepted for a poster presentation and abstract at MHSRS 2025 (Military Health System Research Symposium), the premier U.S. forum for military medical research. This milestone marks further validation of R327G’s potential to transform infection control in military medicine.